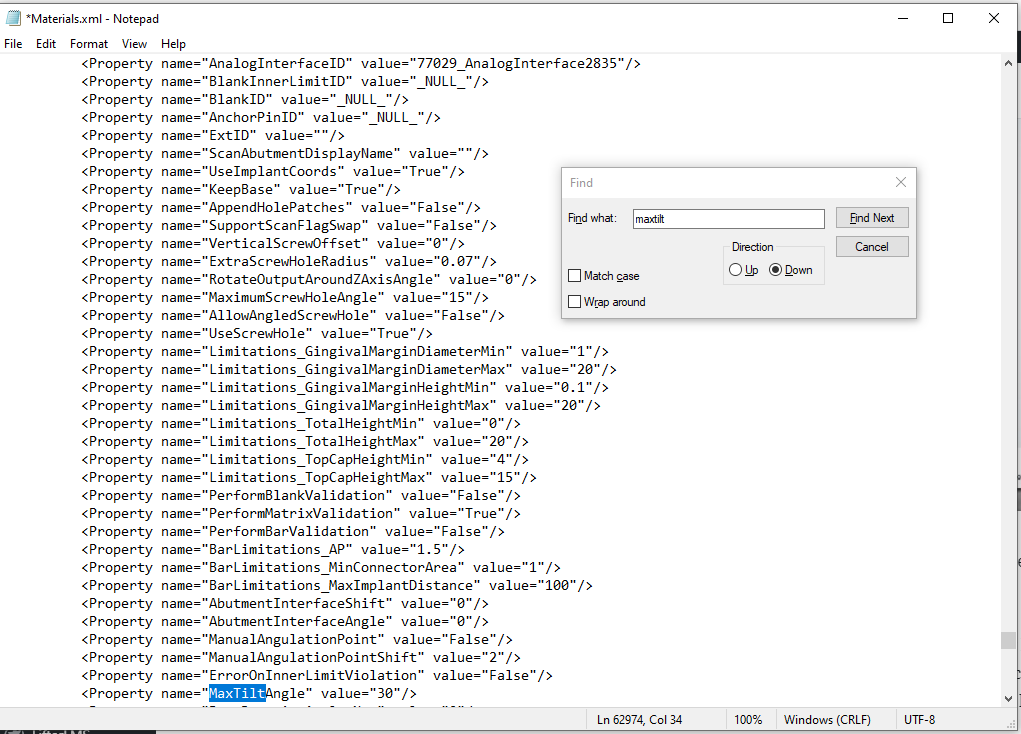
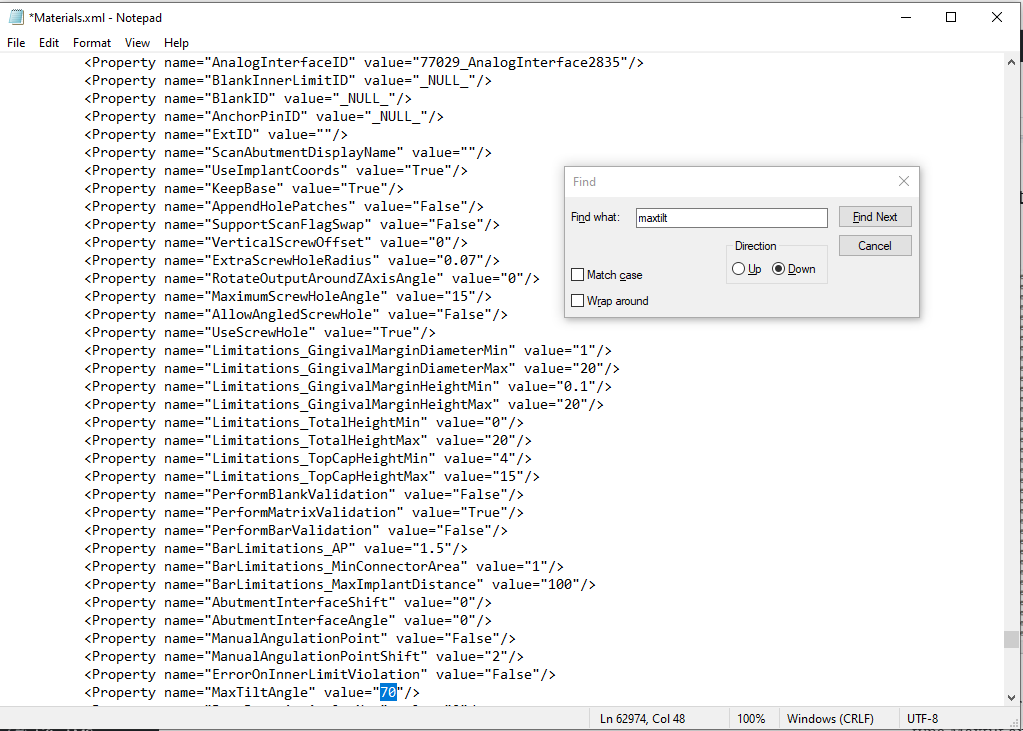
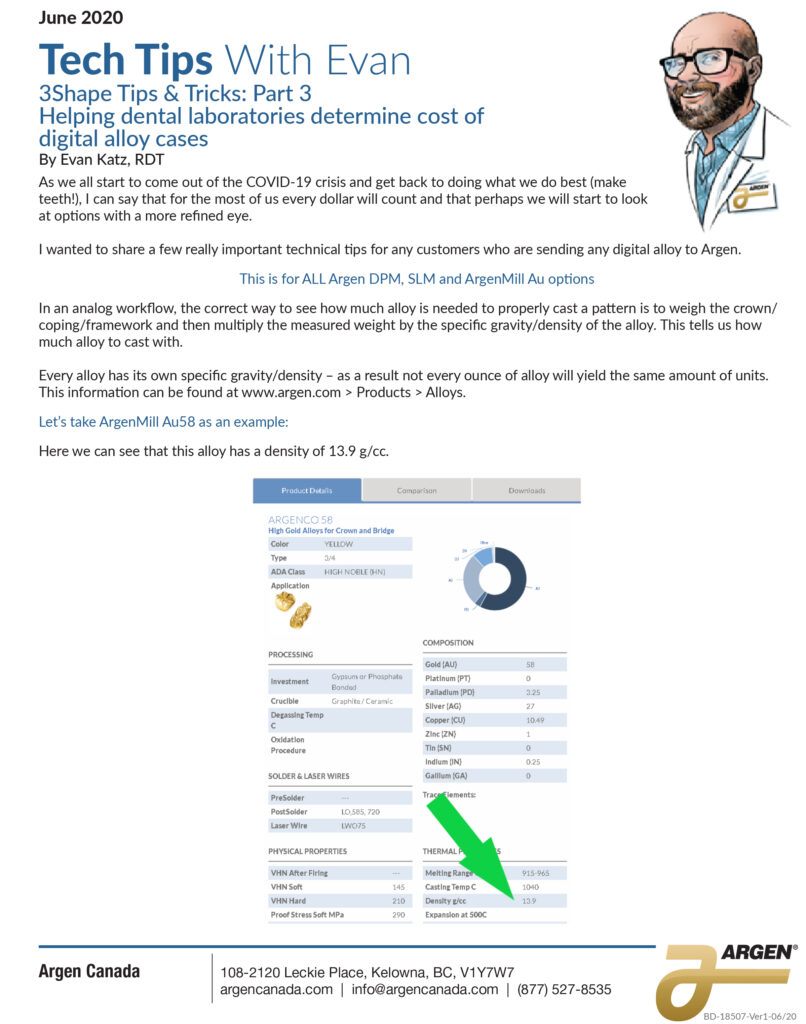
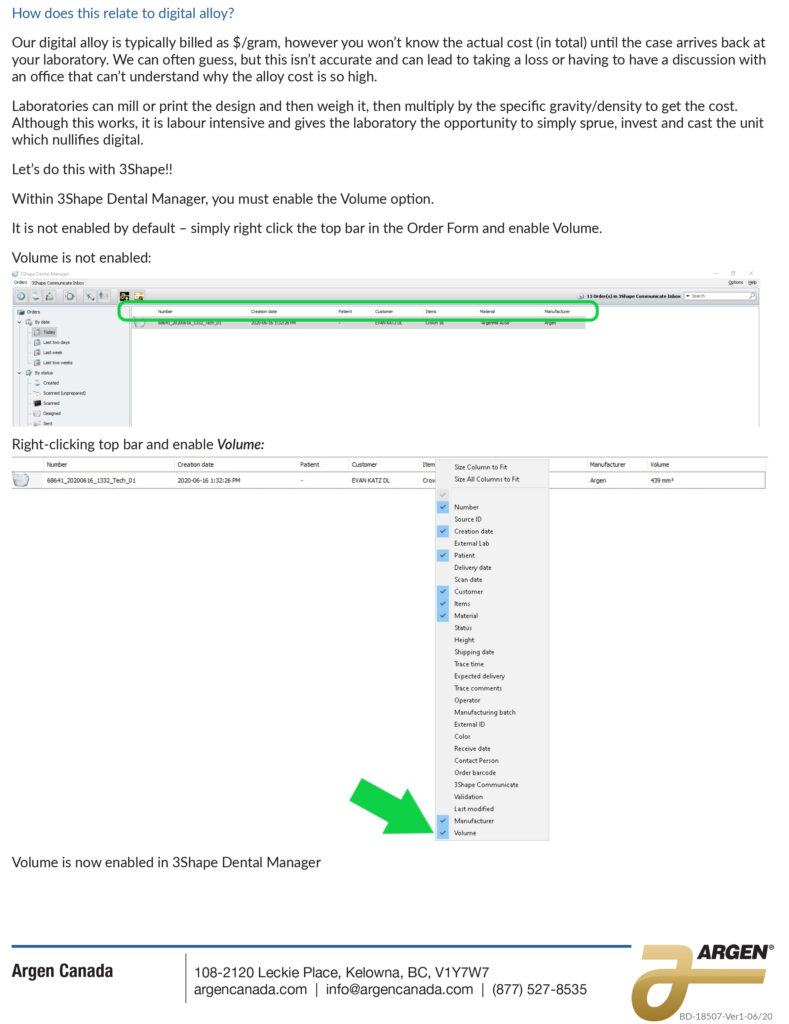
Digital dentistry has evolved from its beginning in the late 1990s. It started with the development of CNC machined crowns derived from digital scans of stone impressions and, by the mid-2000s, progressed to direct digital scans used to generate templates for thermoformed oral appliances. Current practice includes the direct fabrication of surgical guides and dentures and the promise of direct fabrication of aligners using 3D printing.
Carbon’s journey in this space began in 2017 with the release of DPR 10, a stone-colored material for rapid and accurate printing of dental models, with properties suitable for thermoforming models. This was followed by validation of third-party dental materials for surgical guides, custom trays, gingiva masks, and most recently, denture bases and denture teeth for printing on Carbon M Series printers. The growth of Carbon’s user base in dental and orthodontic laboratories has been nothing short of phenomenal. With that growth has come an awareness of the complexity of the regulatory landscape for dental materials, and for our customers’ readiness to meet those requirements. The goal of this white paper is to share our understanding of the current regulatory landscape of 3D printed dental devices.
This white paper is not intended as a substitute for obtaining your own advice regarding legal and regulatory matters, including all FDA regulatory matters. Carbon is not providing you any such advice in connection with this white paper, but rather a basic outline of the regulatory environment. The circumstances of any particular participant in this environment can vary widely, and you should consult your own advisors to obtain advice specific to your circumstances.
KNOW THE FDA BASICS
In general, the FDA does not regulate or approve materials, it regulates devices. Based on risk (Class I, II, or III), the FDA can clear a medical device for sale if (I) it demonstrates safety and is manufactured according to an appropriate quality management system, (II) it can be shown to be equivalent to an existing cleared medical device, or (III) can approve if it is a higher risk device that has had its safety and effectiveness established through more extensive pre-clinical and clinical studies. Since few dental devices fall into the third category, we will focus this white paper on low-to-moderate risk devices (the first and second categories).
Low-risk devices (no need to demonstrate equivalence)
Deemed as Class I, these devices cover items such as toothbrushes, and in digital dentistry include surgical guides, custom trays, and the like. For this level of risk the manufacturer only needs to have in place a quality management system (manufactured under current Good Manufacturing Processes, cGMP), and have data on the safety of the device (generally biocompatibility and mechanical performance data). The manufacturer (or a distributor) is required to register with the FDA and list the device(s).
Moderate risk devices (must demonstrate equivalence)
Deemed as Class II, these devices include denture bases, temporary crowns and bridges, mouth guards, and aligners. For these devices, the manufacturer or distributor must prove their device is “substantially equivalent” to a legally marketed device which has the same purpose (indication for use). Proving that equivalence is described below.
The 510(k) process (how to demonstrate equivalence)
Proving to the FDA that a new medical device is equivalent to an existing medical device (called a predicate device) is referred to as the “510(k) Clearance Process.” Here “equivalence” means the device must treat the same condition in the same way, and its manufacture or composition must not raise any new questions of safety and efficacy. The establishment of “substantial equivalence” (SE) to a “predicate device” allows a manufacturer to legally market their device in the US. Class I devices are exempt from the 510(k) requirements (Figure 1).
There are a few Class II devices that have been “down-regulated” and are exempt from the 510(k) process (“Class II exempt”). An example of this would be 3D printed denture teeth (FDA product code PZY). There are also a few devices that did not receive a risk category in 1976 when the Medical Device Amendments of the Food, Drug and Cosmetics Act were enacted. These fall under the “Unclassified” category, and most devices require 510(k) clearance for marketing (Figure 1). Relevant examples would be a mouthguard prescribed for migraine reduction (FDA product code OCO) or an over-the-counter mouthguard for treating tooth grinding (FDA product code OBR).

Figure 1: Simple process flow showing different FDA clearance requirements for a single “clear” material used for making different dental devices.
FDA APPROVAL: WHAT DOES IT MEAN FOR DIFFERENT STAKEHOLDERS?
First, the word approval applies to high risk medical devices. All lower risk devices are “cleared.” A claim of a dental material having “approval” by the FDA generally means that its been 510(k) cleared for some indication. Clearance for one indication by the FDA or any other regulatory body (for example a Class IIa designation of a device in the EU) does not carry over to any other indications for use.
We’ve said that the FDA does not regulate materials. Yet, it regulates the materials used for 3D printing of dental devices. The following quotes from FDA should also help clarify this fact.
“The materials that the dental branch clears are, in fact, finished devices that are patient matched at the point of care.”
“Dental materials are finished because, in their current state, they are capable of functioning when patient-matched by the end user.”
So, even though they are not the finished medical device, their purpose is to generate a patient-matched medical device. Hence the FDA’s decision to regulate the “starting material.”
What about manufacturers or distributors? Are they regulated?
A manufacturer or distributor of a 3D printing material capable of fabricating a finished device is regulated. A distributor could bring a device not cleared in the US into commerce in the US, but they would then have all the regulatory responsibilities (registration and listing, and if the device is Class II, submission of a 510(k) premarket clearance request).
What about the laboratories? Are they regulated?
This is a complex question. At Carbon, we are learning more and more about laboratories being FDA audited to see if they are adhering to good manufacturing practices. Laboratories have been exempt from registration but have been expected to be compliant with 21 CFR 820 (the FDA Quality Management Systems regulations). But as laboratories engage in more CAD/CAM and use regulated products, including regulated design software, they can veer into the realm of a manufacturer. If a laboratory modifies a design or uses software in conjunction with a material or a printer not validated by the software manufacturer (see below), or manufactures an oral appliance with a material not cleared for that end use, then the laboratory might be creating a misbranded (not appropriate for its indicated use) or adulterated (does not comply with appropriate standards) product.
To be in compliance with 21 CFR 820, a dental laboratory needs to be able to demonstrate to the FDA that it has in place current Good Manufacturing Practices (cGMP). Its beyond the scope of this white paper to describe these requirements, but there are many consulting services available to help dental laboratories put the required systems in place.
What about 3D printers? Are they regulated?
3D printers are not regulated because they are not finished medical devices. However, since the FDA regulates software capable of designing finished medical devices, the software developers work with both laboratories and printer manufacturers to validate the printers, materials, and post-processing ability to produce the finished device with required accuracy and final physical and safety properties.
KEY TAKEAWAYS
Going forward, laboratories need to carefully consider their use of a 3D printing material for the manufacture of dental appliances. Using a dental material to fabricate a device for which it is not FDA cleared can potentially be of risk to patients and can open the lab to regulatory penalties. A material certified for use in Europe (Class IIa) might not be in the US, and vice versa. If you manage a laboratory, follow the guidelines on the label for each material, and get reliable regulatory advice to see if you need to register with the FDA. Manufacturers and distributors need to ensure their materials have the necessary approvals in the regions where these resins are sold.
Dental and orthodontic professionals, software developers, material developers, and printer developers are creating an increasing number of devices to enhance and improve patient oral health care. Carbon is proud to be a leader in this activity and your trusted partner on this journey.
For additional information on Carbon’s dental solution, send us a note at dental@carbon3d.com.